

The electronegativity of tin is 1.96 on the Pauling scale.The first ionization energy of tin is 7.344 eV.In this alloy the proportion of tin is 12% and that of copper is 88%. Tin is alloyed with copper to produce a new alloy called bronze.Below 13 ☌, tin turns into its allotrope called “alpha tin”, and it behaves as a semiconductor.Chemical properties of TinĬhemical properties of tin are mentioned below. Tin has many stable isotopes, but out of them 120Sn is the most abundant (around 32.5%).The crystal structure of tin is tetragonal.The melting point of tin is 231.9 ☌ and its boiling point is 2602 ☌.The atomic mass of tin is 118.71 u and its density is 7.31 g/cm 3.When tin is polished, it produces a shiny surface.Tin is a malleable metal and hence it can be drawn into thin sheets.
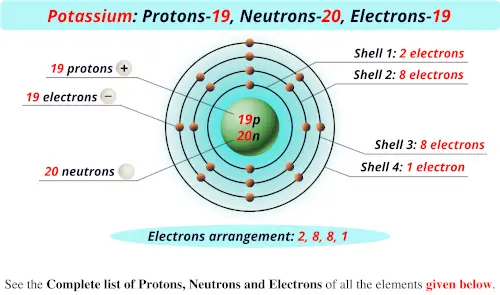
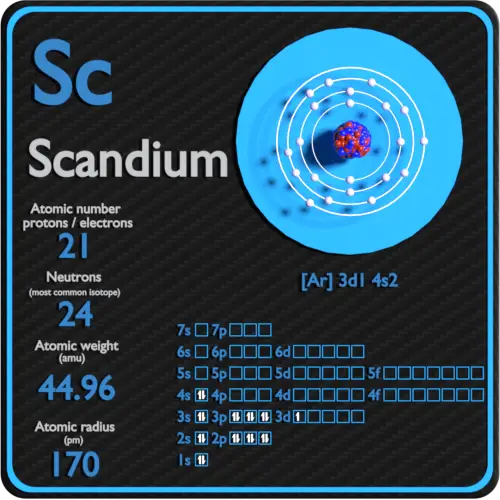
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.įor example the electron configuration of tin is 4d 10 5s 2 5p 2. How can you determine the blocks-wise position of elements? Before knowing this reason, first of all I want to ask you a simple question.


 0 kommentar(er)
0 kommentar(er)
